

You can:
| Name | Beta-1 adrenergic receptor |
|---|---|
| Species | Rattus norvegicus (Rat) |
| Gene | Adrb1 |
| Synonym | adrenergic receptor beta1-adrenoceptor Beta-1 adrenoreceptor Beta-1 adrenoceptor beta-1 adrenergic receptor [ Show all ] |
| Disease | N/A for non-human GPCRs |
| Length | 466 |
| Amino acid sequence | MGAGALALGASEPCNLSSAAPLPDGAATAARLLVLASPPASLLPPASEGSAPLSQQWTAGMGLLLALIVLLIVVGNVLVIVAIAKTPRLQTLTNLFIMSLASADLVMGLLVVPFGATIVVWGRWEYGSFFCELWTSVDVLCVTASIETLCVIALDRYLAITLPFRYQSLLTRARARALVCTVWAISALVSFLPILMHWWRAESDEARRCYNDPKCCDFVTNRAYAIASSVVSFYVPLCIMAFVYLRVFREAQKQVKKIDSCERRFLTGPPRPPSPAPSPSPGPPRPADSLANGRSSKRRPSRLVALREQKALKTLGIIMGVFTLCWLPFFLANVVKAFHRDLVPDRLFVFFNWLGYANSAFNPIIYCRSPDFRKAFQRLLCCARRAACRRRAAHGDRPRASGCLARAGPPPSPGAPSDDDDDDAGATPPARLLEPWAGCNGGTTTVDSDSSLDEPGRQGFSSESKV |
| UniProt | P18090 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | N/A |
| 3D structure model | No available structures or models |
| BioLiP | N/A |
| Therapeutic Target Database | N/A |
| ChEMBL | CHEMBL3252 |
| IUPHAR | N/A |
| DrugBank | N/A |
| Name | propranolol |
|---|---|
| Molecular formula | C16H21NO2 |
| IUPAC name | 1-naphthalen-1-yloxy-3-(propan-2-ylamino)propan-2-ol |
| Molecular weight | 259.349 |
| Hydrogen bond acceptor | 3 |
| Hydrogen bond donor | 2 |
| XlogP | 3.0 |
| Synonyms | Inderex AKOS016050338 KBio2_005083 NCGC00015798-07 Avlocardyl [ Show all ] |
| Inchi Key | AQHHHDLHHXJYJD-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 |
| PubChem CID | 4946 |
| ChEMBL | CHEMBL27 |
| IUPHAR | 564 |
| BindingDB | 25761 |
| DrugBank | DB00571 |
Structure | 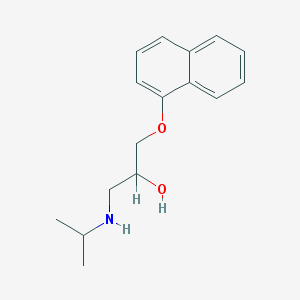 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| ED30 | <1000.0 ug kg-1 | PMID2866246 | ChEMBL |
| ED50 | 0.071 mg.kg-1 | PMID2866246 | ChEMBL |
| Inhibition | -1.16 % | Med Chem Res, (2013) 22:3:1057 | ChEMBL |
| Inhibition | 49.7 % | PMID23538234 | ChEMBL |
| Inhibition | 63.91 % | Med Chem Res, (2013) 22:3:1057 | ChEMBL |
| Inhibition | 73.8 % | PMID23538234 | ChEMBL |
| Kapp | 0.011 uM | PMID37339 | ChEMBL |
| Kd | 2.399 nM | PMID9572886 | ChEMBL |
| Kd | 2.4 nM | PMID9572886 | BindingDB |
| Kd | 4.365 nM | PMID2884312 | ChEMBL |
| Kd | 4.37 nM | PMID2884312 | BindingDB |
| Kd | 4.4 nM | PMID2884312 | BindingDB |
| Kd | 2630.0 nM | PMID9572886 | BindingDB |
| Kd | 2630.27 nM | PMID9572886 | ChEMBL |
| KD' | 2e-09 M | PMID3031293 | ChEMBL |
| Ki | 0.69 nM | PMID1968985 | BindingDB |
| Ki | 3.0 nM | , Med Chem Res, (2008) 17:8:507 | BindingDB,ChEMBL |
| Ki | 3.162 nM | PMID16759089 | ChEMBL |
| Ki | 7.0 nM | PMID27692547 | BindingDB |
| Ki | 7.0 nM | PMID27692547 | ChEMBL |
| Ki | 10.0 nM | PMID16759089 | ChEMBL |
| Ki | 31.62 nM | PMID16759089 | ChEMBL |
| Ki | 39.81 nM | PMID16759089 | ChEMBL |
| Ki | 81.28 nM | PMID1968985 | BindingDB |
| Ki | 199.53 nM | PMID16759089 | ChEMBL |
| Ki | 9700.0 nM | PMID19131251 | BindingDB,ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218