

You can:
| Name | Melanocortin receptor 4 |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | MC4R |
| Synonym | MC4-R MC4 receptor |
| Disease | Obesity; Sexual dysfunction Obesity; Diabetes Obesity Metabolic disorders Sexual dysfunction [ Show all ] |
| Length | 332 |
| Amino acid sequence | MVNSTHRGMHTSLHLWNRSSYRLHSNASESLGKGYSDGGCYEQLFVSPEVFVTLGVISLLENILVIVAIAKNKNLHSPMYFFICSLAVADMLVSVSNGSETIVITLLNSTDTDAQSFTVNIDNVIDSVICSSLLASICSLLSIAVDRYFTIFYALQYHNIMTVKRVGIIISCIWAACTVSGILFIIYSDSSAVIICLITMFFTMLALMASLYVHMFLMARLHIKRIAVLPGTGAIRQGANMKGAITLTILIGVFVVCWAPFFLHLIFYISCPQNPYCVCFMSHFNLYLILIMCNSIIDPLIYALRSQELRKTFKEIICCYPLGGLCDLSSRY |
| UniProt | P32245 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | P32245 |
| 3D structure model | This predicted structure model is from GPCR-EXP P32245. |
| BioLiP | N/A |
| Therapeutic Target Database | T72458 |
| ChEMBL | CHEMBL259 |
| IUPHAR | 285 |
| DrugBank | N/A |
| Name | THIQ |
|---|---|
| Molecular formula | C33H41ClN6O2 |
| IUPAC name | (3R)-N-[(2R)-3-(4-chlorophenyl)-1-[4-cyclohexyl-4-(1,2,4-triazol-1-ylmethyl)piperidin-1-yl]-1-oxopropan-2-yl]-1,2,3,4-tetrahydroisoquinoline-3-carboxamide |
| Molecular weight | 589.181 |
| Hydrogen bond acceptor | 5 |
| Hydrogen bond donor | 2 |
| XlogP | 5.9 |
| Synonyms | (R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid [(R)-1-(4-chloro-benzyl)-2-(4-cyclohexyl-4-[1,2,4]triazol-1-ylmethyl-piperidin-1-yl)-2-oxo-ethyl]-amide KB-70894 1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid [(R)-1-(4-chloro-benzyl)-2-(4-cyclohexyl-4-[1,2,4]triazol-1-ylmethyl-piperidin-1-yl)-2-oxo-ethyl]-amide N-[(1R)-1-[(4-Chlorophenyl)methyl]-2-[4-cyclohexyl-4-(1H-1,2,4-trazol-1-ylmethyl)-1-piperidinyl]-2-oxoethyl]-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide 3-IsoquinolinecarboxaMide,N-[(1R)-1-[(4-chlorophenyl)Methyl]-2-[4-cyclohexyl-4-(1H-1,2,4-triazol-1-ylMethyl)-1-piperidinyl]-2-oxoethyl]-1,2,3,4-tetrahydro-,(3R)- [ Show all ] |
| Inchi Key | HLCHESOMJVGDSJ-LOYHVIPDSA-N |
| Inchi ID | InChI=1S/C33H41ClN6O2/c34-28-12-10-24(11-13-28)18-30(38-31(41)29-19-25-6-4-5-7-26(25)20-36-29)32(42)39-16-14-33(15-17-39,21-40-23-35-22-37-40)27-8-2-1-3-9-27/h4-7,10-13,22-23,27,29-30,36H,1-3,8-9,14-21H2,(H,38,41)/t29-,30-/m1/s1 |
| PubChem CID | 9938402 |
| ChEMBL | CHEMBL339053 |
| IUPHAR | 1338 |
| BindingDB | 50119368 |
| DrugBank | N/A |
Structure | 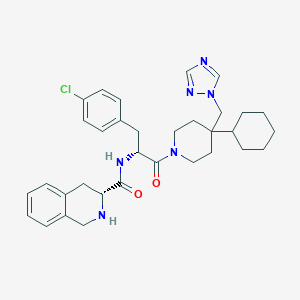 |
| Lipinski's druglikeness | This ligand is heavier than 500 daltons. This ligand has a partition coefficient log P greater than 5. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| %max | 97.0 % | PMID16364639 | ChEMBL |
| Activation | 97.0 % | PMID12361385 | ChEMBL |
| Activity | 16.0 % | PMID16451057 | ChEMBL |
| Activity | 68.0 % | PMID16451057 | ChEMBL |
| Activity | 79.0 % | PMID16451057 | ChEMBL |
| Activity | 89.0 % | PMID16451057 | ChEMBL |
| Activity | 97.0 % | PMID12361385 | ChEMBL |
| Activity | 99.0 % | PMID16451057 | ChEMBL |
| Activity | 104.0 % | PMID16451057 | ChEMBL |
| Activity | 110.0 % | PMID16451057 | ChEMBL |
| Activity | 111.0 % | PMID16451057 | ChEMBL |
| Activity | 118.0 % | PMID16451057 | ChEMBL |
| Activity | 132.0 % | PMID16451057 | ChEMBL |
| EC50 | <1000.0 nM | PMID16451057 | ChEMBL |
| EC50 | 0.61 nM | PMID16451057 | ChEMBL |
| EC50 | 0.71 nM | PMID16451057 | ChEMBL |
| EC50 | 0.82 nM | PMID16451057 | ChEMBL |
| EC50 | 1.0 nM | PMID18078748 | BindingDB,ChEMBL |
| EC50 | 1.2 nM | PMID16451057 | ChEMBL |
| EC50 | 1.45 nM | PMID15615531 | ChEMBL |
| EC50 | 1.5 nM | PMID15615531, PMID16451057 | BindingDB,ChEMBL |
| EC50 | 2.1 nM | PMID16364639, PMID12361385, PMID18078748 | BindingDB,ChEMBL |
| EC50 | 3.6 nM | PMID14643322 | BindingDB,ChEMBL |
| EC50 | 4.0 nM | PMID14552781 | BindingDB,ChEMBL |
| EC50 | 5.7 nM | PMID15357964 | BindingDB,ChEMBL |
| EC50 | 8.5 nM | PMID16451057 | ChEMBL |
| EC50 | 12.0 nM | PMID16451057 | ChEMBL |
| EC50 | 26.0 nM | PMID16451057 | ChEMBL |
| EC50 | 41.0 nM | PMID16451057 | ChEMBL |
| EC50 | 49.0 nM | PMID15615531 | BindingDB,ChEMBL |
| IA | 100.0 % | PMID15357964 | ChEMBL |
| IC50 | 1.2 nM | PMID16364639, PMID15951175, PMID12361385, PMID15615531 | BindingDB,ChEMBL |
| IC50 | 1.26 nM | PMID12361385 | IUPHAR |
| IC50 | 9.0 nM | PMID14643322 | BindingDB,ChEMBL |
| Intrinsic activity | 78.0 % | PMID15615531 | ChEMBL |
| Intrinsic activity | 100.0 % | PMID15615531 | ChEMBL |
| Ki | 0.05 nM | PMID15317471 | ChEMBL |
| Ki | 3.0 nM | PMID18078748 | BindingDB,ChEMBL |
| Ki | 3.2 nM | PMID16451057 | ChEMBL |
| Ki | 4.6 nM | PMID16451057 | ChEMBL |
| Ki | 5.0 nM | PMID16451057 | ChEMBL |
| Ki | 6.7 nM | PMID16451057 | ChEMBL |
| Ki | 8.5 nM | PMID16451057 | ChEMBL |
| Ki | 9.0 nM | PMID15911261 | BindingDB,ChEMBL |
| Ki | 9.8 nM | PMID15615531 | ChEMBL |
| Ki | 10.7 nM | PMID15743921 | BindingDB |
| Ki | 20.0 nM | PMID16451057 | ChEMBL |
| Ki | 33.0 nM | PMID15357964 | BindingDB,ChEMBL |
| Ki | 38.0 nM | PMID16451057 | ChEMBL |
| Ki | 50.0 nM | PMID16451057 | ChEMBL |
| Ki | 80.0 nM | PMID15317471 | ChEMBL |
| Ki | 81.0 nM | PMID16451057 | ChEMBL |
| Ki | 129.0 nM | PMID16451057 | ChEMBL |
| Ki | 646.0 nM | PMID15615531 | BindingDB,ChEMBL |
| Stimulation | 100.0 % | PMID14552781 | ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218