

You can:
| Name | Histamine H4 receptor |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | HRH4 |
| Synonym | Pfi-013 SP9144 HH4R H4R H4 receptor [ Show all ] |
| Disease | Allergic rhinitis Asthma Inflammatory disease Rheumatoid arthritis |
| Length | 390 |
| Amino acid sequence | MPDTNSTINLSLSTRVTLAFFMSLVAFAIMLGNALVILAFVVDKNLRHRSSYFFLNLAISDFFVGVISIPLYIPHTLFEWDFGKEICVFWLTTDYLLCTASVYNIVLISYDRYLSVSNAVSYRTQHTGVLKIVTLMVAVWVLAFLVNGPMILVSESWKDEGSECEPGFFSEWYILAITSFLEFVIPVILVAYFNMNIYWSLWKRDHLSRCQSHPGLTAVSSNICGHSFRGRLSSRRSLSASTEVPASFHSERQRRKSSLMFSSRTKMNSNTIASKMGSFSQSDSVALHQREHVELLRARRLAKSLAILLGVFAVCWAPYSLFTIVLSFYSSATGPKSVWYRIAFWLQWFNSFVNPLLYPLCHKRFQKAFLKIFCIKKQPLPSQHSRSVSS |
| UniProt | Q9H3N8 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | Q9H3N8 |
| 3D structure model | This predicted structure model is from GPCR-EXP Q9H3N8. |
| BioLiP | N/A |
| Therapeutic Target Database | T26500 |
| ChEMBL | CHEMBL3759 |
| IUPHAR | 265 |
| DrugBank | BE0000146 |
| Name | CHEMBL509961 |
|---|---|
| Molecular formula | C22H22ClN5S |
| IUPAC name | N-(1-benzothiophen-2-ylmethyl)-6-chloro-2-(4-methylpiperazin-1-yl)quinazolin-4-amine |
| Molecular weight | 423.963 |
| Hydrogen bond acceptor | 6 |
| Hydrogen bond donor | 1 |
| XlogP | 5.3 |
| Synonyms | BDBM50412483 VUF-10510 |
| Inchi Key | BBGGFNJGPUEVOH-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C22H22ClN5S/c1-27-8-10-28(11-9-27)22-25-19-7-6-16(23)13-18(19)21(26-22)24-14-17-12-15-4-2-3-5-20(15)29-17/h2-7,12-13H,8-11,14H2,1H3,(H,24,25,26) |
| PubChem CID | 25178556 |
| ChEMBL | CHEMBL509961 |
| IUPHAR | N/A |
| BindingDB | 50412483 |
| DrugBank | N/A |
Structure | 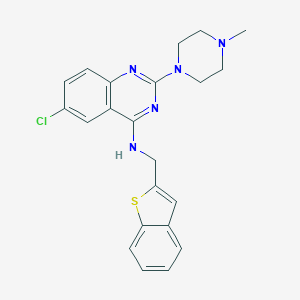 |
| Lipinski's druglikeness | This ligand has a partition coefficient log P greater than 5. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Ki | 269.15 nM | PMID19053770 | BindingDB,ChEMBL |
| Ki | 562.34 nM | PMID20192225 | BindingDB,ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218