

You can:
| Name | Adenosine receptor A1 |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | ADORA1 |
| Synonym | RDC7 A1 receptor A1-AR A1R adenosine receptor A1 |
| Disease | Cardiac arrhythmias Hypertension Cardiac disease Cognitive disorders Diabetes [ Show all ] |
| Length | 326 |
| Amino acid sequence | MPPSISAFQAAYIGIEVLIALVSVPGNVLVIWAVKVNQALRDATFCFIVSLAVADVAVGALVIPLAILINIGPQTYFHTCLMVACPVLILTQSSILALLAIAVDRYLRVKIPLRYKMVVTPRRAAVAIAGCWILSFVVGLTPMFGWNNLSAVERAWAANGSMGEPVIKCEFEKVISMEYMVYFNFFVWVLPPLLLMVLIYLEVFYLIRKQLNKKVSASSGDPQKYYGKELKIAKSLALILFLFALSWLPLHILNCITLFCPSCHKPSILTYIAIFLTHGNSAMNPIVYAFRIQKFRVTFLKIWNDHFRCQPAPPIDEDLPEERPDD |
| UniProt | P30542 |
| Protein Data Bank | 6d9h, 5n2s |
| GPCR-HGmod model | P30542 |
| 3D structure model | This structure is from PDB ID 6d9h. |
| BioLiP | BL0385576, BL0417675 |
| Therapeutic Target Database | T88714, T92072 |
| ChEMBL | CHEMBL226 |
| IUPHAR | 18 |
| DrugBank | BE0000013 |
| Name | N6-Cyclopentyladenosine |
|---|---|
| Molecular formula | C15H21N5O4 |
| IUPAC name | (2R,3R,4S,5R)-2-[6-(cyclopentylamino)purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol |
| Molecular weight | 335.364 |
| Hydrogen bond acceptor | 8 |
| Hydrogen bond donor | 4 |
| XlogP | 0.9 |
| Synonyms | (2R,3R,4S,5R)-2-[6-(cyclopentylamino)purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol AC1LCWG1 CTK1D5898 MFCD00036845 n6-cyclopentyl-adenosine [ Show all ] |
| Inchi Key | SQMWSBKSHWARHU-SDBHATRESA-N |
| Inchi ID | InChI=1S/C15H21N5O4/c21-5-9-11(22)12(23)15(24-9)20-7-18-10-13(16-6-17-14(10)20)19-8-3-1-2-4-8/h6-9,11-12,15,21-23H,1-5H2,(H,16,17,19)/t9-,11-,12-,15-/m1/s1 |
| PubChem CID | 657378 |
| ChEMBL | CHEMBL68738 |
| IUPHAR | 380 |
| BindingDB | 25400 |
| DrugBank | N/A |
Structure | 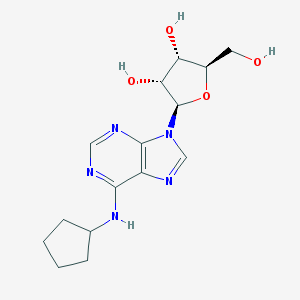 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Activity | 51.0 % | PMID18258439 | ChEMBL |
| Change in cAMP | -100.0 % | PMID15771447 | ChEMBL |
| EC50 | 0.19 nM | Bioorg. Med. Chem. Lett., (1996) 6:7:811 | ChEMBL |
| EC50 | 0.19 nM | N/A | BindingDB |
| EC50 | 4.15 nM | PMID10841798 | ChEMBL |
| EC50 | 4.2 nM | PMID10841798 | BindingDB |
| EC50 | 6.3 nM | PMID22738238 | BindingDB,ChEMBL |
| IC50 | 2.7 nM | PMID18042389 | BindingDB,ChEMBL |
| Inhibition | 69.0 % | PMID11520205 | ChEMBL |
| Inhibition | 100.0 % | PMID22921089 | ChEMBL |
| Intrinsic activity | 1.0 - | PMID18042389 | ChEMBL |
| Ki | 0.398108 - 316.228 nM | PMID7798201, PMID9920910, PMID15476669, PMID16444290, PMID15740718, PMID9827575, PMID16518376 | IUPHAR |
| Ki | 0.45 nM | PMID16366590 | BindingDB,ChEMBL |
| Ki | 1.8 nM | PMID22921089 | BindingDB,ChEMBL |
| Ki | 2.25 nM | PMID17933541 | BindingDB,ChEMBL |
| Ki | 2.3 nM | , None | BindingDB,ChEMBL |
| Ki | 5.9 nM | PMID26356532 | BindingDB,ChEMBL |
| Ki | 9.0 nM | PMID18783211 | BindingDB |
| Ki | 10.0 nM | PMID18258439, PMID18637670, PMID15771447 | BindingDB,ChEMBL |
| Ki | 21.5 nM | PMID18042389 | BindingDB,ChEMBL |
| Max | 100.0 % | PMID10841798 | ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218