
You can:
| Name | Proteinase-activated receptor 2 |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | F2RL1 |
| Synonym | Protease-activated receptor-2 PAR2 PAR-2 GPR11 G-protein coupled receptor 11 [ Show all ] |
| Disease | N/A |
| Length | 397 |
| Amino acid sequence | MRSPSAAWLLGAAILLAASLSCSGTIQGTNRSSKGRSLIGKVDGTSHVTGKGVTVETVFSVDEFSASVLTGKLTTVFLPIVYTIVFVVGLPSNGMALWVFLFRTKKKHPAVIYMANLALADLLSVIWFPLKIAYHIHGNNWIYGEALCNVLIGFFYGNMYCSILFMTCLSVQRYWVIVNPMGHSRKKANIAIGISLAIWLLILLVTIPLYVVKQTIFIPALNITTCHDVLPEQLLVGDMFNYFLSLAIGVFLFPAFLTASAYVLMIRMLRSSAMDENSEKKRKRAIKLIVTVLAMYLICFTPSNLLLVVHYFLIKSQGQSHVYALYIVALCLSTLNSCIDPFVYYFVSHDFRDHAKNALLCRSVRTVKQMQVSLTSKKHSRKSSSYSSSSTTVKTSY |
| UniProt | P55085 |
| Protein Data Bank | 5ndz, 5ndd |
| GPCR-HGmod model | P55085 |
| 3D structure model | This structure is from PDB ID 5ndz. |
| BioLiP | BL0377325, BL0377326 |
| Therapeutic Target Database | N/A |
| ChEMBL | CHEMBL5963 |
| IUPHAR | 348 |
| DrugBank | N/A |
| Name | CHEMBL3752956 |
|---|---|
| Molecular formula | C26H35FN4O4 |
| IUPAC name | N-[(2S)-3-cyclohexyl-1-[[(2S,3S)-1-[(3-fluorophenyl)methylamino]-3-methyl-1-oxopentan-2-yl]amino]-1-oxopropan-2-yl]-1,2-oxazole-5-carboxamide |
| Molecular weight | 486.588 |
| Hydrogen bond acceptor | 6 |
| Hydrogen bond donor | 3 |
| XlogP | 5.1 |
| Synonyms | N/A |
| Inchi Key | GJBUNTXVUMKFQT-HYVJGQCMSA-N |
| Inchi ID | InChI=1S/C26H35FN4O4/c1-3-17(2)23(26(34)28-16-19-10-7-11-20(27)14-19)31-24(32)21(15-18-8-5-4-6-9-18)30-25(33)22-12-13-29-35-22/h7,10-14,17-18,21,23H,3-6,8-9,15-16H2,1-2H3,(H,28,34)(H,30,33)(H,31,32)/t17-,21-,23-/m0/s1 |
| PubChem CID | 56639888 |
| ChEMBL | CHEMBL3752956 |
| IUPHAR | N/A |
| BindingDB | N/A |
| DrugBank | N/A |
Structure | 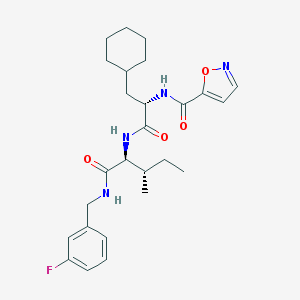 |
| Lipinski's druglikeness | This ligand has a partition coefficient log P greater than 5. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Activity | 28.0 % | PMID26725028 | ChEMBL |
| Inhibition | 74.0 % | PMID26725028 | ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218