
You can:
| Name | C5a anaphylatoxin chemotactic receptor 1 |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | C5AR1 |
| Synonym | complement component 5a receptor 1 complement C5a receptor C5A CD88 C5R1 [ Show all ] |
| Disease | Vasculitis Atopic dermatitis Inflammatory disease Rheumatoid arthritis |
| Length | 350 |
| Amino acid sequence | MDSFNYTTPDYGHYDDKDTLDLNTPVDKTSNTLRVPDILALVIFAVVFLVGVLGNALVVWVTAFEAKRTINAIWFLNLAVADFLSCLALPILFTSIVQHHHWPFGGAACSILPSLILLNMYASILLLATISADRFLLVFKPIWCQNFRGAGLAWIACAVAWGLALLLTIPSFLYRVVREEYFPPKVLCGVDYSHDKRRERAVAIVRLVLGFLWPLLTLTICYTFILLRTWSRRATRSTKTLKVVVAVVASFFIFWLPYQVTGIMMSFLEPSSPTFLLLKKLDSLCVSFAYINCCINPIIYVVAGQGFQGRLRKSLPSLLRNVLTEESVVRESKSFTRSTVDTMAQKTQAV |
| UniProt | P21730 |
| Protein Data Bank | 6c1r, 6c1q, 5o9h |
| GPCR-HGmod model | P21730 |
| 3D structure model | This structure is from PDB ID 6c1r. |
| BioLiP | BL0415511, BL0415514, BL0415513, BL0415512, BL0401194,BL0401195,BL0401196, |
| Therapeutic Target Database | T15439 |
| ChEMBL | CHEMBL2373 |
| IUPHAR | 32 |
| DrugBank | N/A |
| Name | CHEMBL538918 |
|---|---|
| Molecular formula | C27H32ClN3O |
| IUPAC name | (E)-3-(2-chlorophenyl)-N-[2-methyl-4-(octylamino)quinolin-6-yl]prop-2-enamide |
| Molecular weight | 450.023 |
| Hydrogen bond acceptor | 3 |
| Hydrogen bond donor | 2 |
| XlogP | 7.9 |
| Synonyms | (E)-N-(4-Octylamino-2-methyl-6-quinolinyl)-2-chlorocinnamamide SCHEMBL8352423 |
| Inchi Key | CWQLRNHZQNXHEL-DTQAZKPQSA-N |
| Inchi ID | InChI=1S/C27H32ClN3O/c1-3-4-5-6-7-10-17-29-26-18-20(2)30-25-15-14-22(19-23(25)26)31-27(32)16-13-21-11-8-9-12-24(21)28/h8-9,11-16,18-19H,3-7,10,17H2,1-2H3,(H,29,30)(H,31,32)/b16-13+ |
| PubChem CID | 15133407 |
| ChEMBL | CHEMBL538918 |
| IUPHAR | N/A |
| BindingDB | N/A |
| DrugBank | N/A |
Structure | 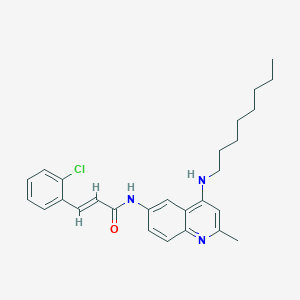 |
| Lipinski's druglikeness | This ligand has a partition coefficient log P greater than 5. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| IC50 | <100.0 ug.mL-1 | PMID1310118 | ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218